Multinational clinical trials initiative
Helping researchers in different countries collaborate on multinational clinical trials that might not be feasible in a single country or with support from a single funder
The next expression of interest deadline for the multinational clinical trials initiative is 09:00 BST, Friday 22nd May 2026.
What is the GCRFF multinational clinical trials initiative?
The multinational clinical trials initiative is a subgroup of the GCRFF whose aim is to help increase the likelihood of academic-led, multinational cardiovascular clinical trials securing the funding they need to be delivered successfully. It is made up of the following members:
The Initiative helps researchers from different countries collaborate on plans, and coordinate and optimise funding applications, for ambitious, multinational cardiovascular clinical trials that might not be feasible in a single country or with support from a single funder.
The aim is to see trials delivered faster, with more generalisable results, at a more affordable cost to national funders. Ultimately, this could lead to findings that save and improve the lives of people with cardiovascular disease throughout the world.
Related links
Expression of interest (EOI) portal
Funding schemes for member organisations
Active trials endorsed by the GCRFF
Frequently asked questions
How it works
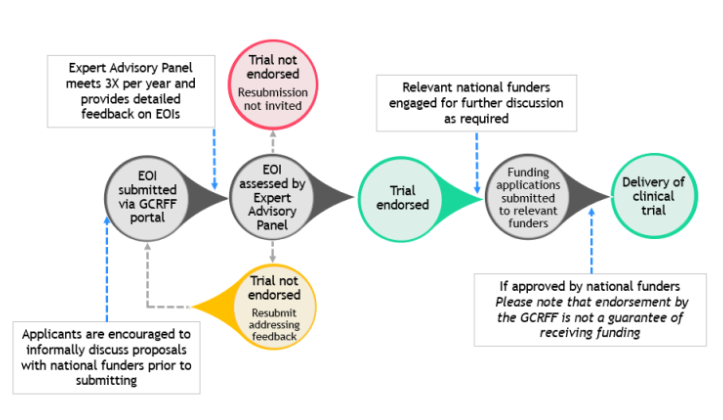
Investigators wishing to submit a proposal for the initiative should follow the following process:
A team of investigators planning a multinational cardiovascular clinical trial can submit an initial proposal (an Expression of Interest [EOI]) for the proposal through a dedicated portal, before making any funding applications.
EOIs must meet specified eligibility criteria to be considered, and investigators should read how to apply and our FAQs prior to submission.
The EOIs are considered by the GCRFF’s Expert Advisory Panel (EAP), which meets three times per year.
Trials considered to have merit will be endorsed by the panel. Endorsement of an EOI by the GCRFF is an indication of strong support by the GCRFF members for the proposed trial. The EAP also provides feedback on the design of the trial and its need for multinational funding. Funders involved in the initiative may also attend EAP meetings, and can indicate, within an EAP meeting or after convening within their organization, whether they would be willing to receive an application for the trial. View the review form to see how EOIs are assessed and scored by the EAP.
Investigators in each country collaborating on a trial can then prepare coordinated but individual applications to their country’s funders (GCRFF or non GCRFF members), requesting support for the relevant national component of the trial. The applications to national funders are considered through the funders’ usual application and peer review processes. The applications to funders should include the letter of endorsement from the EAP. View information about relevant funding schemes for clinical trials for member organisations.
The scientific rationale and trial design is expected to be harmonised across all applications (e.g. the inclusion criteria, intervention and control and primary endpoint should be the same across all countries involved).
The idea is that having input from the panel into the proposed trial and its design before investigators start applying for funding, plus being able to share that the trial was endorsed by the GCRFF in any funding applications, will help increase the chances of a positive decision by national funders. It also helps that potential funders know the level of support requested from other funders, providing greater assurance that the trial will be deliverable and costs will be shared.
Please note: While endorsement of a trial by the GCRFF is intended to demonstrate strong support for the trial, it does not guarantee funding of the proposal by individual funders.
Information for applicants
-
The principal investigator should be a senior researcher working in an established institution eligible to hold grant funding in a country where a funder that is part of the GCRFF Multinational Clinical Trials Initiative is based (see list). They must have a strong track record of grant support, and an internationally recognised research profile.
The trial should plan to test specific interventions or pathways of care for the prevention, diagnosis or treatment of heart and circulatory diseases. Interventions include drugs, surgery, devices, psychological, physical and educational interventions.
The trial should address an unmet clinical need of importance to people affected by, or at risk of, cardiovascular disease. Its results should have the potential to change clinical practice.
There should be a clear need for a multinational trial to answer the clinical question.
The investigators should be planning to conduct the trial and apply for funding in at least three countries where funders that are members of the GCRFF Multinational Clinical Trials Initiative are based (see list).
The investigators can be planning to apply for funding from both GCRFF and non GCRFF member funders (investigators are encouraged to explore co-funding beyond the GCRFF members)
The EOI must include co-applicants from the countries of the national funders who have been named in the funding request. Co-applicants should have been fully engaged in the development of the study, should have inputted into the EOI, and (should the EOI be endorsed) be planning to be fully engaged with the process of applying for funding in their respective countries.
It should be clear why the trial should be considered for support by national cardiovascular funders and not by others (e.g. pharma).
Investigators are asked to provide an estimated total cost for the trial, and indicative amounts that will be requested from each proposed funder in subsequent funding applications. While a detailed breakdown of costings is not required at this stage, the indicative costings should be a reasonable estimate of the funding required to successfully deliver the trial, and the amounts attributed to the individual funders should be realistic. The Expert Advisory Panel will take into account whether a trial potentially offers value for money when making endorsement decisions.
Early phase studies (Phases 1 and 2), or trials that have already started in one or more countries are not eligible for the scheme. The remit of the initiative is to help facilitate the development of later phase, definitive clinical trials.
Investigators are encouraged to informally discuss their trial proposal with the relevant national funders before submitting an EOI to the GCRFF Multinational Clinical Trials Initiative.
If you plan to apply to the DZHK for funding, you must check your eligibility with the DZHK head office before submitting to the GCRFF.
If you plan to apply to the DHF for funding, you must submit a short pre-application to the DHF before submitting to the GCRFF.
-
If you would like to see what information you will need to provide before you start to apply, view an example Expression of Interest (EOI) form.
To apply, you will need to login to the portal via the BHF Grants Management System.
New users will need to register for an account before they can submit an EOI. Please use the academic email address provided by your institution as your contact email. If your email needs to be changed, or if it is not possible for you to use an academic address, please contact GCRFFtrials@bhf.org.uk.
Once you have established an account, sign in to the application portal and select ‘My Applications’ from the top right of the screen.
Select ‘Available grants’ from the menu on the left and scroll down to GCRFF Multinational Clinical Trials Expression of Interest. Click ‘Start’ to begin an EOI.
If not already recorded on their portal profile, applicants will be asked to provide contact information and institution details. All pages of the form will then need to be completed - clicking ‘Submit application’ on the summary page will then submit the EOI for consideration by the GCRFF.
Receipt of your submission will be acknowledged by email automatically and followed up with a further email indicating when an outcome of the application can be expected.
-
There are three submission rounds a year for the Multinational Clinical Trials Initiative. The next panel dates and submission deadlines are provided below:
March 2026
Expression of Interest submission deadline – 09:00 GMT, Friday 23rd January 2026
Expert Advisory Panel meeting – 19th March 2026
July 2026
Expression of Interest submission deadline – 09:00 BST, Friday 22nd May 2026
Expert Advisory Panel meeting – 16th July 2026
November 2026
Expression of Interest submission deadline – 09:00 BST, Friday 25th September 2026
Expert Advisory Panel meeting – 24th November 2026
-
Expression of Interest are assessed by an Expert Advisory Panel, including independent members nominated by each of the funders involved in the GCRFF Multinational Clinical Trials Initiative. View the current membership of the EAP.
The panel meets three times per year in March, July and November.
The panel serves in an advisory capacity only, deciding whether or not to endorse the EOI.
There is no funding available through the GCRFF Multinational Clinical Trials Initiative.
Please note: while endorsement of a trial by the GCRFF is intended to demonstrate strong support for the trial, it does not guarantee funding of the proposal.
Individual members of the EAP are unable to enter into dialogue with researchers regarding specific Expressions of Interest. Researchers should under no circumstances contact members of the EAP in this regard. Any queries about decisions made by the EAP should be directed to the secretariat or the relevant funder’s administrative team.
-
If your Expression of Interest is endorsed by the GCRFF Multinational Clinical Trials Initiative Expert Advisory Panel, you should apply for support for the proposed clinical trial through the national funders' usual funding mechanisms. Please note that while endorsement is intended to demonstrate support for applications to individual funders, it is not a guarantee of receiving funding if an application is made.
Principal investigators of endorsed trials will also be asked to complete a brief annual progress report on the submission portal (starting one year after the trial is endorsed). This will help the secretariat track follow on applications to national funders and will assist the funders in evaluating the GCRFF Multinational Clinical Trials Initiative. The report will ask for details about the status of individual applications to funders, including those originally listed in the EOI, and any additional applications. You can also keep us informed of any key developments as they occur, such as the result of a funding application, by emailing GCRFFtrials@bhf.org.uk.
The metrics of success for the Initiative will be the proportion of endorsed proposals that are able to secure funding for their clinical trial, and ultimately whether the trials are successfully delivered and influence clinical practice.
For specific advice from individual GCRFF Funders and to view information about relevant clinical trial funding schemes, please click here.
-
The GCRFF Multinational Clinical Trials Initiative has endorsed trials encompassing a broad range of cardiovascular diseases since it was set up in 2021. Read more about active clinical trials endorsed by the GCRFF.
-
For more information, please consult the Frequently asked questions page.
Get in touch
This GCRFF workstream is led by British Heart Foundation. For any other queries about the multinational clinical trials initiative and the application process, please contact GCRFFtrials@bhf.org.uk.