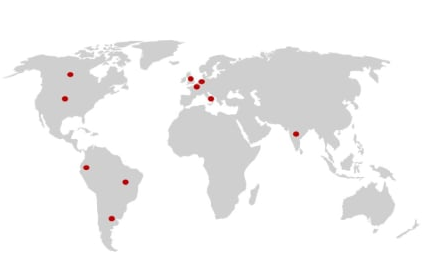
Active GCRFF trials
Active trials endorsed by the GCRFF Multinational Clinical Trials Initiative
ASPIRING - Antiplatelet Secondary Prevention International Randomised trial after INtracerebral haemorrhaGe
LEADER-PAD - Low dose ColchicinE in pAtients with peripheral artery DiseasE to address residual vascular Risk
CONTEMP-ICD - The Comparative Effectiveness of Contemporary Heart Failure Medical Management With vs. Without an ICD
EAST-STROKE - Early treatment of Atrial fibrillation for Stroke prevention Trial in acute STROKE
ROMA-Women - Randomized comparison of the Outcomes of single vs Multiple Arterial grafts trial in Women
WARRIORS - Women’s abdominal aortic Aneurysm Research: Immediate Repair or Routine Surveillance
EAST-High - Early atrial fibrillation ablation for stroke prevention in patients with high comorbidity burden
ASPIRING - Antiplatelet Secondary Prevention International Randomised trial after INtracerebral haemorrhaGe
Led by Professor Rustam Salman, University of Edinburgh (UK)
Endorsed November 2021
EOI reference: GCRFF/21/270024
Total sample size: 4148 participants
Estimated total cost: £4.3m
Trial registration: ISRCTN16705062
Recruitment planned in:
UK (2828 participants), Canada (450 participants), The Netherlands (356 participants), Australia (330 participants), Belgium (240 parictipants)
Supported by:
BHF (international co-ordination and recruitment in the UK), Canadian Institutes of Health Research (recruitment in Canada), Dutch Heart Foundation (recruitment in the Netherlands), Medical Research Futures Fund (recruitment in Australia), The Research Foundation Flanders (recruitment in Belgium)
An intracerebral haemorrhage (or ICH) is a type of stroke caused by bleeding into the brain. People who survive an ICH are at an increased risk of experiencing another major vascular event (such as a heart attack, stroke, or dying due to a clotting or bleeding problem) in the future. Antiplatelet drugs, such as aspirin, help to prevent dangerous blood clots and are widely used to help prevent heart attacks and strokes in people at high risk. Currently, people who have experienced an ICH are usually kept off drugs that prevent clotting because of the potential risk of further bleeding into the brain. However, a previous clinical trial called RESTART showed that taking antiplatelet drugs after an ICH seemed to be safe, and might even help to prevent major vascular events, such as heart attacks or strokes caused by a blood clot. Building on these findings, the team behind RESTART are now leading a larger, international clinical trial called ASPIRING. ASPIRING will involve 4148 people who have experienced an ICH, who will be recruited from hospitals across the UK and at least four other countries worldwide. Participants will be randomly assigned to either start or avoid taking an antiplatelet drug - this can be aspirin, clopidogrel, dipyridamole, cilostazol, ticagrelor or ticlopidine - depending on what their doctor thinks is best for them. They will be followed up for up to 5 years to find out whether there is a difference in the number of major vascular events between the two groups. If taking an antiplatelet is found to be beneficial, this could help improve outcomes for the quarter of a million ICH survivors worldwide each year.
LEADER-PAD - Low dose ColchicinE in pAtients with peripheral artery DiseasE to address residual vascular Risk
Led by Dr Noel Chan, McMaster University (Canada)
Endorsed November 2021
EOI reference: GCRFF/21/270035
Total sample size: 6150 participants
Estimated total cost: £11.3m
Trial registration: NCT04774159, ISRCTN99954716
Recruitment planned in:
Canada, Ecuador, Argentina, India, Belgium, Italy, Brazil (3000 participants), UK (1500 participants), The Netherlands (1500 participants), United States of America (1000 participants)
Supported by funders:
Canadian Institutes of Health Research (for international co-ordination and recruitment in Canada, Ecuador, Argentina, India, Belgium, Italy, Brazil), British Heart Foundation (recruitment in the UK), ZonMw (recruitment in the Netherlands), National Heart, Lung, and Blood Institute (recruitment in the USA)
Peripheral arterial disease (PAD) happens when the arteries supplying blood to the arms or legs become narrowed. It is usually caused by inflammation and fatty build up in the walls of the arteries (atherosclerosis), meaning less blood can get through, similar to the blockage of blood vessels supplying the heart in people with coronary artery disease (CAD). PAD usually effects the legs, and can often lead to leg pain and, in severe cases, gangrene and even amputation. People with PAD are also at high risk of other cardiovascular complications, such as a heart attack or stroke, even if they are treated with existing preventive medications (such as anti-clotting drugs or statins). Colchicine is an anti-inflammatory medication commonly used to treat gout. Previous research has shown that a low dose of colchicine could help to reduce heart attacks, stroke and other cardiovascular complications for people with CAD. As PAD has the same underlying cause as CAD, an international team of researchers want to find out whether colchicine could help people living with PAD too. The LEADER-PAD trial will involve 6150 participants with PAD worldwide. All participants will take colchicine for a two week ‘run in’ period, to make sure that they are able to safely take the drug (as it can have side effects, such as digestive symptoms). After run-in, they will be randomly assigned to take either a low dose of colchicine (0.5mg per day) or an identical placebo tablet that doesn’t have any drug in it. They will attend hospital for follow up visits every 6 months for up to 3 years to find out whether there is a difference in the number of major vascular or limb events between the two groups (including dying due to a cardiovascular cause, heart attack, stroke, and severe clotting in a limb – including needing an amputation). If the results of LEADER-PAD show that taking colchicine is beneficial, the drug would be a new treatment for a group of patients who do not currently have many effective medications available. As colchicine is a cheap drug, which is already safely used for other conditions, this would also mean it could be rolled out more quickly as a new treatment option worldwide.
CONTEMP-ICD - The Comparative Effectiveness of Contemporary Heart Failure Medical Management With vs. Without an ICD
Led by Dr Ilan Goldenberg, University of Rochester (USA)
Endorsed November 2021
EOI reference: GCRFF/21/270056
Total sample size: 3290 participants
Estimated total cost: ~$27m (USD)
Trial registration: NCT06543446
Recruitment planned in:
USA, Canada (up to 3290 participants), Slovenia (200 participants)
Supported by:
Patient-Centred Outcomes Research Institute (recruitment in US and Canada)
Some people with heart failure are at risk of experiencing fast, dangerous heart rhythms. An ICD is a device that is implanted under the skin in the chest to monitor the heartbeat, and if a dangerous rhythm is detected, deliver an electric shock to try and restore a normal heart rhythm. ICDs can be lifesaving, but many people who receive an ICD will never need it to deliver a shock, and having an ICD has some risks (such as infections or shocks being given inappropriately). Also, the current guidelines on recommending ICDs are mostly based on trials that happened before many newer heart failure medications were available. This has called into question whether people with heart failure, who have not experienced a life-threatening heart rhythm problem in the past, should be offered an ICD. The CONTEMP-ICD trial aims to enrol 3290 people with heart failure, who are eligible to have an ICD under the current guidelines, but have been assessed as being at low risk of needing an ICD shock using a clinical scoring system. Participants will be randomly assigned to either receive an ICD or not. All participants will be given recommended heart failure medication. They will be followed up for up to 4 years to assess whether there is any difference in outcomes between the two groups (including risk of dying, experiencing a ‘cardiovascular event’ such as a heart attack or stroke, and ICD complications such as inappropriate shocks). The results of CONTEMP-ICD will provide vital information on whether the guidelines on ICD should be adjusted in light of advances in heart failure medications.
EAST-STROKE - Early treatment of Atrial fibrillation for Stroke prevention Trial in acute STROKE
Led by Professor Dr Götz Thomalla, University Medical Center Hamburg-Eppendorf (Germany)
Endorsed March 2022
EOI reference: GCRFF/22/270081
Total sample size: 1746 participants
Estimated total cost: ~£10m
Trial registration: NCT05293080
Recruitment planned in:
Germany, Australia, Brazil, Canada, Spain, Switzerland, Netherlands, UK
Supported by:
Horizon Europe, DFG (recruitment in Germany), Medical Research Futures Fund (recruitment in Australia), Swiss National Science Foundation (recruitment in Switzerland)
Atrial fibrillation (AF) is a common abnormal heart rhythm, where the heart beats irregularly and often too fast. Having AF can cause blood clots to form in the heart, which can travel to the brain and lead to an ischaemic stroke. Around 20% of ischaemic strokes are linked to AF, and people with AF who have a stroke are at high risk of having another one. Current treatment of AF in people who have had a stroke usually includes taking anti-clotting medication, management of cardiovascular risk factors and ‘rate control’ - where people are given medications to slow their heart rate). But there is another way of treating AF called rhythm control - where people are given medications or have an ablation or cardioversion procedure to help restore a normal heart rhythm. A previous clinical trial called EAST-AFNET-4 found that earlier rhythm control treatment can lower the risk of stroke, heart attacks and other heart problems. However, it remains unclear whether early rhythm control is beneficial for people with AF who have had a stroke, as only 8% of participants in EAST-AFNET-4 had a ischaemic stroke before joining the trial. The EAST-STROKE trial aims to address this evidence gap by enrolling up to 1746 people with AF diagnosed in the past year, who have had an ischaemic stroke in the last 4 weeks. Participants will be randomly assigned to receive early rhythm control (an ablation or starting anti-arrhythmic medication within 4 weeks) or usual care. The main outcome the researchers will assess is if there is any difference in the number of participants who have another stroke, die due to a heart-related reason, or are hospitalised due to heart failure or heart attack between the groups. The results have the potential to change how AF is treated following a stroke and to help prevent many thousands of new strokes.
ROMA-Women - Randomized comparison of the Outcomes of single vs Multiple Arterial grafts trial in Women
Led by Dr Mario Gaudino, Weill Cornell Medicine (USA)
Endorsed July 2022
EOI reference: GCRFF/21/270098
Total sample size: 2000 participants
Estimated total cost: ~£10m
Trial registration: NCT04124120
Recruitment planned in:
USA (812 participants), Canada (130 participants), UK (216 participants), Germany (170 participants), Austria (90 participants), The Netherlands (250 participants)
Supported by:
Canadian Institutes of Health Research (recruitment in Canada), British Heart Foundation (recruitment in the UK), DZHK (recruitment in Germany), Austrian Science Fund (recruitment in Austria), Starr Foundation (support for overall trial), Dutch Heart Foundation (recruitment in The Netherlands), National Heart Lung and Blood Institute (recruitment in the USA)
Coronary artery bypass grafting (CABG) is surgery used to treat people with coronary heart disease. It usually involves taking an artery that supplies blood to the inner chest and using it to bypass a narrowed area of coronary artery supplying the heart. Surgeons then use additional vessel ‘grafts’ (usually pieces of artery from the arm, or vein from the leg) as needed. There’s some evidence that - when multiple grafts are needed - it’s better if multiple artery grafts (MAG) are used, rather than a single arterial graft (SAG) and additional veins. This is because grafted veins are more likely to become blocked or diseased over time than arteries. But despite these potential benefits, currently in the UK MAG is only used for about 9% of men and 7% of women undergoing CABG. It’s a more technically difficult surgery to do – particularly in women, who tend to be physically smaller than men – and has also been linked to an increased risk of complications from the surgical wound. Previous randomised clinical trials (the gold standard way of testing a treatment) have not shown a clear difference in longer term outcomes between people who received MAG or SAG, but less than 20% of people who took part in previous trials were women. The team behind ROMA-Women want to address this knowledge gap by carrying out the first ever heart surgery trial to involve only women. They aim to enrol 2000 women undergoing CABG, who will be randomly assigned to receive either MAG or SAG during their surgery and followed up for up to 4 years (looking for any difference in the risk of dying, having a heart attack or stroke, needing a repeat surgery or hospitalisation between the two). If the results are positive, ROMA-Women could lead to a change in clinical guidelines, more women receiving MAG, and improved outcomes for women undergoing CABG. As CABG is the most commonly performed heart surgery in adults worldwide, the findings have the potential to improve the health of many millions of women.
News about ROMA-Women
ROMA:Women: Innovative Approaches for the First Cardiac Surgery Trial in Women > Circulation. 2023
ROMA:Women-a trial dedicated to women to improve coronary bypass outcomes > J Thorac Cardiovasc Surg. 2024
Randomized Trials in Cardiac Surgery: Why and How > Eur J Cardiothorac Surg. 2025
WARRIORS - Women’s abdominal aortic Aneurysm Research: Immediate Repair or Routine Surveillance
Led by Mr Colin Bicknell (co-CI: Miss Anna-Louise Pouncey), Imperial College London (UK)
Endorsed July 2022
EOI reference: GCRFF/22/27009
Total sample size: 1112 participants
Estimated total cost: ~£9m
Trial registration: NCT06394271
Recruitment planned in:
UK, Australia, Belgium, Canada, Denmark, Germany, Finland, France, Hungary, Ireland, Italy, Latvia, Netherlands, New Zealand, Norway, Portugal, Sweden, Serbia, USA
Supported by:
British Heart Foundation (international co-ordination and recruitment in UK), Dutch Heart Foundation (recruitment in the Netherlands and Belgium), Independent Research Fund Denmark (recruitment in Denmark), Swedish Heart and Lung Foundation (recruitment in Sweden), National Heart, Lung, and Blood Institute (recruitment in the USA), New Zealand Health Research Council (recruitment in New Zealand), Novo Nordisk, Medtronic, Terumo, Canadian Vascular Society, Camelia Botnar Arterial Research Foundation, German Society for Vascular Surgery & Vascular Medicine, University of Manitoba
The aorta is a large artery that carries oxygen-rich blood away from your heart to the rest of your body. The part that passes through the abdomen is called the abdominal aorta, and is usually about 2cm wide. An AAA is a balloon-like swelling of the abdominal aorta, which if it grows large enough can burst, causing internal bleeding that can be fatal. AAAs can be repaired by having a procedure called endovascular aneurysm repair (EVAR), where a tube stent is inserted into the enlarged area of the abdominal aorta, reinforcing it and helping to prevent it from rupturing. People with an AAA have its size and growth monitored with regular ultrasound scans and are considered for having the AAA repaired if it reaches 5.5cm wide. However, this threshold for deciding when to intervene is based on previous clinical trials that involved very few women. Women’s arteries tend to be smaller than men’s arteries, and AAAs in women may be more likely to rupture at smaller diameters compared to me. There is a clear need to find out what threshold for repair should be used for women with an AAA. The WARRIORS trial will involve 1112 women with an AAA between 4-5.4cm wide, which is not causing them any symptoms. Participants will be randomly assigned to either have early EVAR, or to continue with standard ultrasound monitoring of their AAA and have EVAR if it reaches 5.5cm wide or they experience a rupture. They will be followed up for 5 years to monitor whether there is any difference in the risk of AAA-related death or AAA rupture between the two groups. The research team will also look for any differences in health-related quality of life and carry out a healthcare cost analysis, to help assess whether offering earlier repair would be cost-effective for the NHS. WARRIORS will help enable the development of sex-specific guidelines for when to repair AAAs and address an area where there is currently a lack of evidence to support treatment in women.
EAST-High- Early atrial fibrillation ablation for stroke prevention in patients with high comorbidity burden
Led by Professor Dr Paulus Kirchhof, University Medical Center Hamburg-Eppendorf (Germany)
Endorsed November 2022
EOI reference: GCRFF/22/270114
Total sample size: 1746 participants
Estimated total cost: ~£10m
Trial registration: NCT06324188
Recruitment planned in:
Germany, Canada, Czechia, Hungary, Poland, Spain, Netherlands, UK
Supported by:
Else Kröner-Fresenius-Stiftung (recruitment in Germany), Accelerating Clinical Trials (pilot phase in Canada), British Heart Foundation (recruitment in the UK), Dutch Heart Foundation (recruitment in the Netherlands), Medtronic (to support countries without public funding and co-support countries with public funding)
Atrial fibrillation (AF) is a common condition where the heart beats irregularly and often too fast, increasing the risk of stroke and heart problems. This risk is higher in older individuals and those with other health conditions, such as high blood pressure or diabetes. It’s been estimated that over half of people newly diagnosed with AF have multiple health conditions. Ablation is a treatment for AF, that involves creating small areas of scarring in the heart to help block abnormal electrical signals causing an irregular heart rhythm. This procedure can be very effective but is currently less commonly offered to people who are older or have other health conditions. More evidence is needed whether ablation is safe and effective for this group, who have tended to be excluded from previous clinical trials in this area. The EASThigh trial will address this evidence gap. The study aims to enrol 2318 people, who have been diagnosed with AF in the past two years and have other health conditions. Participants will be randomly assigned to either have an ablation within 8 weeks of joining the trial, or standard AF care (which could include a later ablation). If early ablation is found to be safe and effective at reducing the risk of death, stroke or being hospitalised with heart failure, this could lead to more people with AF having access to this potentially beneficial treatment, sooner after diagnosis.